What Is A Fuel Cell?
What Is A Fuel Cell? A Fuel Cell utilizes the chemical energy of hydrogen or another fuel to neatly and productively produce power. If hydrogen is the fuel, power, water, and warmth are the lone items. Fuel cells are exceptional as far as the assortment of their possible applications; they can give power to frameworks as extensive as a utility power station and as little as a PC.
Single Answer: A fuel cell is an electrochemical cell that proselytes the chemical energy of a fuel (regularly hydrogen) and an oxidizing specialist into power through a couple of redox reactions. Fuel cells are not the same as most batteries in requiring a constant wellspring of fuel and oxygen to support the chemical response, though in a battery the chemical energy ordinarily comes from metals and their particles or oxides that are usually effectively present in the battery, besides in-stream batteries. Fuel cells can create power persistently however long fuel and oxygen are provided.
The primary fuel cells were designed by Sir William Grove in 1838. The primary business utilization of fuel cells came over a century later after the development of the hydrogen-oxygen fuel cell by Francis Thomas Bacon in 1932. The antacid fuel cell, otherwise called the Bacon fuel cell after its innovator, has been utilized in NASA space programs since the mid-1960s to create power for satellites and space containers.
Also read: What is Holography?
From that point forward, fuel cells have been utilized in numerous different applications. Fuel cells are utilized for essential and reinforcement power for business, modern and private structures, and in distant or blocked off regions. They are additionally used to power fuel cell vehicles, including forklifts, cars, transports, boats, bikes, and submarines.
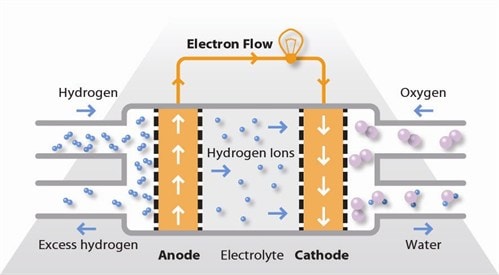
There are numerous kinds of fuel cells, yet they all comprise of an anode, a cathode, and an electrolyte that permits particles, frequently decidedly charged hydrogen particles (protons), to move between the different sides of the fuel cell. At the anode an impetus makes the fuel go through oxidation responses that produce particles and electrons. The particles move from the anode to the cathode through the electrolyte. Simultaneously, electrons stream from the anode to the cathode through an outer circuit, creating direct flow power.
At the cathode, another impetus causes particles, electrons, and oxygen to respond, shaping water and perhaps different items. Fuel cells are grouped by the sort of electrolyte they use and by the distinction in startup time going from 1 second for proton-trade film fuel cells (PEM fuel cells, or PEMFC) to 10 minutes for strong oxide fuel cells (SOFC). A connected innovation is stream batteries, in which the fuel can be recovered by re-energizing.
Singular fuel cells produce moderately little electrical possibilities, about 0.7 volts, so cells are "stacked", or put in arrangement, to make adequate voltage to meet an application's requirements. notwithstanding power, fuel cells produce water, heat, and, contingent upon the fuel source, extremely limited quantities of nitrogen dioxide and different outflows. The energy proficiency of a fuel cell is for the most part somewhere in the range of 40 and 60%; in any case, if squander heat is caught in a cogeneration plot, efficiencies of up to 85% can be obtained.
Also read: What is Graphene?
Fuel cells can be utilized in a wide scope of utilization, including transportation, material dealing with, fixed, compact, and crisis reinforcement power applications. Fuel cells have a few advantages over ordinary ignition-based innovations as of now utilized in many power plants and traveler vehicles. Fuel cells can work at higher efficiencies than burning motors and can change the chemical energy in the fuel over to electrical energy with efficiencies of up to 60%. Fuel cells have lower emanations than ignition motors.
Hydrogen fuel cells emanate just water, so there are no carbon dioxide outflows and no air contaminations that make brown haze and mess wellbeing up at the mark of activity. Additionally, fuel cells hush up during activity as they have fewer moving parts.
Fuel cells work like batteries, yet they don't run down or need re-energizing. They produce power and warmth insofar as fuel is provided. A fuel cell comprises of two terminals—a negative terminal (or anode) and a positive terminal (or cathode)— sandwiched around an electrolyte. A fuel, like hydrogen, is taken care of to the anode, and the air is taken care of to the cathode. In a hydrogen fuel cell, an impetus at the anode isolates hydrogen atoms into protons and electrons, which take various ways to the cathode.
The electrons go through an outside circuit, making a progression of power. The protons move through the electrolyte to the cathode, where they join with oxygen and the electrons to create water and warmth.
History of Fuel Cell
The principal references to hydrogen fuel cells showed up in 1838. In a letter dated October 1838 yet distributed in the December 1838 release of The London and Edinburgh Philosophical Magazine and Journal of Science, Welsh physicist, and counselor Sir William Grove expounded on the improvement of his first rough fuel cells. He utilized a blend of sheet iron, copper, and porcelain plates, and an answer of sulfate of copper and weaken acid.
Also read: What Is Smart Clothing?
In a letter to a similar distribution written in December 1838 yet distributed in June 1839, German physicist Christian Friedrich Schönbein talked about the principal unrefined fuel cell that he had designed. His letter talked about ebb and flow created from hydrogen and oxygen disintegrated in water. Grove later portrayed his plan, in 1842, in a similar diary. The fuel cell he made utilized comparative materials to the present phosphoric corrosive fuel cell.
In 1932, English architect Francis Thomas Bacon effectively fostered a 5 kW fixed fuel cell. The basic fuel cell (AFC), otherwise called the Bacon fuel cell after its innovator, is perhaps the most evolved fuel cell advance, which NASA has utilized since the mid-1960s.
In 1955, W. Thomas Grubb, a scientific expert working for the General Electric Company (GE), further altered the first fuel cell plan by utilizing a sulfonated polystyrene particle trade layer as the electrolyte. After three years another GE physicist, Leonard Niedrach, conceived a method of saving platinum onto the layer, which filled in as an impetus for the important hydrogen oxidation and oxygen decrease responses. This got known as the "Grubb-Niedrach fuel cell". GE proceeded to foster this innovation with NASA and McDonnell Aircraft, prompting its utilization during Project Gemini.
This was the primary business utilization of a fuel cell. In 1959, a group drove by Harry Ihrig fabricated a 15 kW fuel cell farm truck for Allis-Chalmers, which was exhibited across the U.S. at state fairs. This framework utilized potassium hydroxide as the electrolyte and compacted hydrogen and oxygen as the reactants. Later in 1959, Bacon and his partners exhibited a functional five-kilowatt unit fit for powering a welding machine. During the 1960s, Pratt and Whitney authorized Bacon's U.S. licenses for use in the U.S. space program to supply power and drinking water. In 1991, the main hydrogen fuel cell vehicle was created by Roger Billings.
Types of Fuel Cells by design
Fuel cells come in numerous assortments; notwithstanding, they all work in a similarly general way. They are comprised of three adjoining sections: the anode, the electrolyte, and the cathode. Two chemical responses happen at the interfaces of the three distinct sections. The net consequence of the two responses is that fuel is devoured, water or carbon dioxide is made, and an electric ebb and flow is made, which can be utilized to power electrical gadgets, typically alluded to as the heap.
At the anode an impetus oxidizes the fuel, normally hydrogen, transforming the fuel into an emphatically charged particle and a contrarily charged electron. The electrolyte is a substance explicitly planned so particles can go through it, however, the electrons can't. The liberated electrons travel through a wire making the electric flow. The particles go through the electrolyte to the cathode. When arriving at the cathode, the particles are brought together with the electrons and the two respond with a third chemical, normally oxygen, to make water or carbon dioxide.
Proton-exchange membrane fuel cells (PEMFCs)
In the prototype hydrogen–oxide proton-exchange membrane fuel cell plan, a proton-leading polymer layer (commonly Nafion) contains the electrolyte arrangement that isolates the anode and cathode sides. This was known as a strong polymer electrolyte fuel cell (SPEFC) in the mid-1970s before the proton-trade component was surely known.
Also read: What is Inside a Black Hole?
On the anode side, hydrogen diffuses to the anode impetus where it later separates into protons and electrons. These protons regularly respond with oxidants making them become what is usually alluded to as multi-worked with proton layers. The protons are directed through the film to the cathode, yet the electrons are compelled to go in an outside circuit (providing power) because the layer is electrically protecting. On the cathode impetus, oxygen particles respond with the electrons and protons to frame the water.
Notwithstanding this unadulterated hydrogen type, there are hydrocarbon fuels for fuel cells, including diesel, methanol, and chemical hydrides. The side-effects of these kinds of fuel are carbon dioxide and water. At the point when hydrogen is utilized, the CO2 is delivered when methane from petroleum gas is joined with steam, in a cycle called steam methane transforming, to create the hydrogen.
Phosphoric acid fuel cell (PAFC)
Phosphoric acid fuel cells (PAFC) were first planned and presented in 1961 by G. V. Elmore and H. A. Leather treater. In these cells, phosphoric corrosive is utilized as a non-conductive electrolyte to pass positive hydrogen particles from the anode to the cathode. These cells generally work in temperatures of 150 to 200 degrees Celsius. This high temperature will cause warmth and energy misfortune if the warmth isn't eliminated and utilized appropriately.
This warmth can be utilized to create steam for cooling frameworks or some other nuclear power burning-through system. Using this warmth in cogeneration can upgrade the proficiency of phosphoric corrosive fuel cells from 40 to half to about 80%. Phosphoric corrosive, the electrolyte utilized in PAFCs, is a non-conductive fluid corrosive which powers electrons to go from anode to cathode through an outer electrical circuit.
Since the hydrogen particle creation rate on the anode is little, platinum is utilized as an impetus to expand this ionization rate. A vital burden of these cells is the utilization of an acidic electrolyte. This builds the consumption or oxidation of parts presented to phosphoric acid.
Solid acid fuel cell (SAFC)
Solid acid fuel cells (SAFC) are portrayed by the utilization of strong corrosive material as the electrolyte. At low temperatures, strong acids have an arranged sub-atomic design like most salts. At hotter temperatures, some strong acids go through a stage change to turn out to be exceptionally scattered "superprotonic" structures, which expands conductivity by a few significant degrees. The principal confirmation of-idea SAFCs were created in 2000 utilizing cesium hydrogen sulfate (CsHSO4). Current SAFC frameworks use cesium dihydrogen phosphate (CsH2PO4) and have exhibited lifetimes in a large number of hours.
Alkaline fuel cell (AFC)
The antacid fuel cell or hydrogen-oxygen fuel cell was planned and first exhibited openly by Francis Thomas Bacon in 1959. It was utilized as an essential wellspring of electrical energy in the Apollo space program. The cell comprises two permeable carbon cathodes impregnated with an appropriate impetus like Pt, Ag, CoO, and so on The space between the two terminals is loaded up with a concentrated arrangement of KOH or NaOH which fills in as an electrolyte. H2 gas and O2 gas rise into the electrolyte through the permeable carbon terminals.
Also read: Fastest Supercomputers In The World
Subsequently, the general response includes a mix of hydrogen gas and oxygen gas to frame the water. The cell runs constantly until the reactant's inventory is depleted. This sort of cell works proficiently in the temperature range 343–413 K and gives a capability of about 0.9 V. AAEMFC is a kind of AFC that utilizes a strong polymer electrolyte rather than fluid potassium hydroxide (KOH) and it is better than watery AFC.
High-temperature fuel cells
Solid oxide fuel cell
Solid oxide fuel cells utilize a strong material, most regularly a ceramic material called yttria-balanced out zirconia (YSZ), as the electrolyte. Since SOFCs are made completely of strong materials, they are not restricted to the level plane arrangement of different kinds of fuel cells and are regularly planned as moved cylinders. They require high working temperatures (800–1000 °C) and can be run on an assortment of fuels including characteristic gas.
SOFCs are interesting since in those, contrarily charged oxygen particles travel from the cathode (positive side of the fuel cell) to the anode (negative side of the fuel cell) rather than emphatically charged hydrogen particles heading out from the anode to the cathode, just like the case in any remaining kinds of fuel cells. Oxygen gas is taken care of through the cathode, where it ingests electrons to make oxygen particles. The oxygen particles at that point venture out through the electrolyte to respond with hydrogen gas at the anode.
The response at the anode produces power and water as result. Carbon dioxide may likewise be a side-effect contingent upon the fuel, however, the fossil fuel byproducts from a SOFC framework are not exactly those from a petroleum derivative burning plant.
SOFC frameworks can run on fuels other than unadulterated hydrogen gas. Be that as it may, since hydrogen is essential for the responses recorded over, the fuel chose should contain hydrogen particles. For the fuel cell to work, the fuel should be changed over into unadulterated hydrogen gas. SOFCs are prepared to do inside transforming light hydrocarbons like methane (normal gas), propane, and butane. These fuel cells are at the beginning phase of development.
Difficulties exist in SOFC frameworks because of their high working temperatures. One such test is the potential for carbon residue to develop on the anode, which hinders the inside changing interaction. Examination to address this "carbon coking" issue at the University of Pennsylvania has shown that the utilization of copper-based cermet (heat-safe materials made of earthenware and metal) can diminish coking and the deficiency of performance.
Another detriment of SOFC frameworks is moderate beginning uptime, making SOFCs less helpful for versatile applications. Despite these hindrances, a high working temperature gives a benefit by eliminating the requirement for a valuable metal impetus like platinum, consequently decreasing expense. Also, squander heat from SOFC frameworks might be caught and reused, expanding the hypothetical by and large productivity to as high as 80–85%.
The high working temperature is generally because of the actual properties of the YSZ electrolyte. As temperature diminishes, so does the ionic conductivity of YSZ. In this way, to acquire the ideal execution of the fuel cell, a high working temperature is required. As indicated by their site, Ceres Power, a UK SOFC fuel cell maker, has fostered a technique for decreasing the working temperature of their SOFC framework to 500–600 degrees Celsius.
They supplanted the normally utilized YSZ electrolyte with a CGO (cerium gadolinium oxide) electrolyte. The lower working temperature permits them to utilize treated steel rather than ceramic as the cell substrate, which diminishes the cost and start-up season of the system.
Molten-carbonate fuel cell (MCFC)
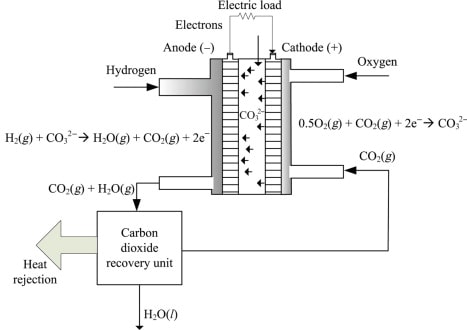
Liquid carbonate fuel cells (MCFCs) require a high working temperature, 650 °C (1,200 °F), like SOFCs. MCFCs use lithium potassium carbonate salt as an electrolyte, and this salt melts at high temperatures, taking into account the development of charge inside the cell – for this situation, negative carbonate ions.
Like SOFCs, MCFCs are equipped for changing petroleum products over to a hydrogen-rich gas in the anode, dispensing with the need to create hydrogen remotely. The transforming interaction makes CO
2 discharges. MCFC-viable fuels incorporate gaseous petrol, biogas, and gas created from coal. The hydrogen in the gas responds with carbonate particles from the electrolyte to create water, carbon dioxide, electrons, and modest quantities of different chemicals. The electrons travel through an outside circuit making power and get back to the cathode. There, oxygen from the air and carbon dioxide reused from the anode respond with the electrons to shape carbonate particles that recharge the electrolyte, finishing the circuit.
Also read: What is a Cryogenic Rocket Engine
Likewise, with SOFCs, MCFC impediments incorporate sluggish beginning up occasions in light of their high working temperature. This makes MCFC frameworks not reasonable for versatile applications, and this innovation will no doubt be utilized for fixed fuel cell purposes. The primary test of MCFC innovation is the cells' short life expectancy. The high-temperature and carbonate electrolyte lead to consumption of the anode and cathode.
These variables speed up the debasement of MCFC parts, diminishing the solidness and cell life. Scientists are resolving this issue by investigating erosion-safe materials for segments just as fuel cell plans that may expand cell existence without diminishing performance.
MCFCs hold a few benefits over other fuel cell advancements, including their protection from pollutions. They are not inclined to "carbon coking", which alludes to carbon development on the anode that outcomes in diminished execution by hindering the inward fuel improving interaction. Hence, carbon-rich fuels like gases produced using coal are viable with the framework.
The United States Department of Energy guarantees that coal, itself, may even be a fuel alternative, later on, accepting the framework can be made impervious to debasements, for example, sulfur and particulates that come about because of changing over coal into hydrogen. MCFCs likewise have moderately high efficiencies. They can arrive at fuel-to-power productivity of half, significantly higher than the 37–42% proficiency of a phosphoric corrosive fuel cell plant. Efficiencies can be however high as 65% when the fuel cell may be matched with a turbine, and 85% if heat is caught and utilized in a consolidated warmth and power (CHP) system.
FuelCell Energy, a Connecticut-based fuel cell producer, creates and sells MCFC fuel cells. The organization says that their MCFC items range from 300 kW to 2.8 MW frameworks that accomplish 47% electrical effectiveness and can use CHP innovation to acquire higher by and large efficiencies. One item, the DFC-ERG, is joined with a gas turbine and, as indicated by the organization, it accomplishes an electrical proficiency of 65%.
Electric storage fuel cell
The electric stockpiling fuel cell is a customary battery chargeable by electric power input, utilizing the traditional electro-chemical impact. Be that as it may, the battery further incorporates hydrogen (and oxygen) contributions for on the other hand charging the battery chemically.
The efficiency of leading fuel cell types
Theoretical maximum efficiency
The energy effectiveness of a framework or gadget that proselytes energy is estimated by the proportion of the measure of helpful energy put out by the framework to the aggregate sum of energy that is placed in or by valuable yield energy as a level of the all-out input energy. On account of fuel cells, valuable yield energy is estimated in electrical energy created by the framework. Information energy is the energy put away in the fuel.
As indicated by the U.S. Branch of Energy, fuel cells are by and large somewhere in the range of 40 and 60% energy efficient. This is higher than some different frameworks for the energy age. For instance, the ordinary inward ignition motor of a vehicle is about 25% energy efficient. In joined warmth and power (CHP) frameworks, the warmth created by the fuel cell is caught and put to utilize, expanding the proficiency of the framework to up to 85–90%.
Also read: What is Blockchain Technology
The hypothetical greatest proficiency of a power age framework is never reached practically speaking, and it doesn't think about different strides in power age, like creation, transportation, and capacity of fuel and change of the power into mechanical power. Be that as it may, this computation permits the examination of various sorts of power age. The hypothetical greatest proficiency of a fuel cell approaches 100%, while the hypothetical most extreme effectiveness of inner ignition motors is around 58%.
Practically speaking
In a fuel-cell vehicle, the tank-to-wheel effectiveness is more prominent than 45% at low loads and shows normal upsides of about 36% when a driving cycle like the NEDC (New European Driving Cycle) is utilized as a test procedure. A similar NEDC incentive for a Diesel vehicle is 22%. In 2008 Honda delivered a show fuel cell electric vehicle (the Honda FCX Clarity) with a fuel stack asserting a 60% tank-to-wheel efficiency.
It is likewise essential to take misfortunes because of fuel creation, transportation, and capacity into account. Fuel cell vehicles running on packed hydrogen may have power-plant-to-wheel productivity of 22% if the hydrogen is put away as high-pressure gas, and 17% on the off chance that it is put away as fluid hydrogen. Fuel cells can't store energy like a battery, besides hydrogen, yet in certain applications, for example, independent power plants dependent on spasmodic sources, for example, sun oriented or wind power, they are joined with electrolyzers and capacity frameworks to shape an energy stockpiling framework.
Starting in 2019, 90% of hydrogen was utilized for oil refining, chemicals, and compost creation, and 98% of hydrogen is delivered by steam methane changing, which produces carbon dioxide. The general effectiveness of such plants, utilizing unadulterated hydrogen and unadulterated oxygen can be "from 35 up to 50 percent", contingent upon the gas thickness and other conditions. The electrolyzer/fuel cell framework can store inconclusive amounts of hydrogen and is along these lines appropriate for long-haul stockpiling.
Strong oxide fuel cells produce heat from the recombination of oxygen and hydrogen. The ceramic can run as hot as 800 degrees Celsius. This warmth can be caught and used to warm water in a miniature joined warmth and power (m-CHP) application. At the point when the warmth is caught, absolute effectiveness can arrive at 80–90% at the unit, however doesn't think about creation and appropriation misfortunes. CHP units are being grown today for the European home market.
Applications
Power
Fixed fuel cells are utilized for business, mechanical, and private essential and reinforcement power ages. Fuel cells are exceptionally helpful as power sources in distant areas, like space apparatus, far-off climate stations, huge parks, interchanges focuses, rustic areas including research stations, and in certain tactical applications. A fuel cell framework running on hydrogen can be conservative and lightweight and have no major moving parts. Since fuel cells have no moving parts and don't include ignition, in ideal conditions they can accomplish up to 99.9999% reliability. This compares to short of one moment of vacation in a six-year period.
Since fuel cell electrolyzer frameworks don't store fuel in themselves, yet rather depend on outer capacity units, they can be effectively applied in huge scope energy stockpiling, country regions being one example. There are various sorts of fixed fuel cells so efficiencies change, yet most are somewhere in the range of 40% and 60% energy efficient. However, when the fuel cell's waste warmth is utilized to warm a structure in a cogeneration framework this effectiveness can increment to 85%. This is essentially more proficient than customary coal power plants, which are just around 33% energy efficient.
Assuming creation at scale, fuel cells could save 20–40% on energy costs when utilized in cogeneration systems. Fuel cells are likewise much cleaner than conventional power age; a fuel cell power plant utilizing gaseous petrol as a hydrogen source would make short of what one ounce of contamination (other than CO2) for each 1,000 kW·h delivered, contrasted with 25 pounds of poisons created by ordinary ignition systems. Fuel Cells additionally produce 97% less nitrogen oxide outflows than regular coal-terminated power plants.
One such test case program is working on Stuart Island in Washington State. There the Stuart Island Energy Initiative has constructed a total, shut circle framework: Solar boards power an electrolyzer, which makes hydrogen. The hydrogen is put away in a 500-U.S.- gallon (1,900 L) tank at 200 pounds for each square inch (1,400 kPa), and runs a ReliOn fuel cell to give full-electric back-up to the off-the-lattice home. Another shut framework circle was divulged in late 2011 in Hempstead, NY.
Fuel cells can be utilized with bad-quality gas from landfills or waste-water treatment plants to create power and lower methane discharges. A 2.8 MW fuel cell plant in California is supposed to be the biggest of the type. Small-scale (sub-5kWhr) fuel cells are being created for use in private off-matrix deployment.
Cogeneration
Joined warmth and power (CHP) fuel cell frameworks, including miniature consolidated warmth and power frameworks are utilized to produce both power and warmth for homes, places of business, and processing plants. The framework creates steady electric power and simultaneously delivers hot air and water from the waste warmth. As an outcome CHP framework can possibly save essential energy as they can utilize squander heat which is by and large dismissed by nuclear power change systems. The ordinary limit scope of the home fuel cell is 1–3 kWel, 4–8 kWth. CHP frameworks connected to ingestion chillers utilize their waste warmth for refrigeration.
The waste warmth from fuel cells can be redirected throughout the late spring straightforwardly into the ground giving further cooling while the waste warmth during winter can be siphoned straightforwardly into the structure. The University of Minnesota possesses the patent rights to this sort of system
Co-age frameworks can arrive at 85% productivity (40–60% electric and the rest of thermal). Phosphoric-corrosive fuel cells (PAFC) contain the biggest fragment of existing CHP items worldwide and can give joined efficiencies near 90%. Molten carbonate (MCFC) and strong oxide fuel cells (SOFC) are likewise utilized for consolidated warmth and power age and have electrical energy efficiencies around 60%. Disadvantages of co-age frameworks incorporate lethargic increase and down rates, significant expense, and short lifetime.
Also, their need to have a boiling water stockpiling tank to streamline the warm warmth creation was a genuine burden in the homegrown commercial center where space in homegrown properties is at an extraordinary premium.
Delta-ee specialists expressed in 2013 that with 64% of worldwide deals the fuel cell miniature consolidated warmth and power passed the customary frameworks in deals in 2012. The Japanese ENE FARM venture will pass 100,000 FC mCHP frameworks in 2014, 34.213 PEMFC and 2.224 SOFC were introduced in the period 2012–2014, 30,000 units on LNG and 6,000 on LPG.
Fuel cell electric vehicles (FCEVs)
Automobiles
By year-end 2019, around 18,000 FCEVs had been rented or sold worldwide. Three fuel cell electric vehicles have been presented for business rent and deal: the Honda Clarity, Toyota Mirai, and the Hyundai ix35 FCEV. Extra show models incorporate the Honda FCX Clarity, and Mercedes-Benz F-Cell. As of June 2011 exhibition FCEVs had driven more than 4,800,000 km (3,000,000 mi), with more than 27,000 refuelings. Fuel cell electric vehicles include a normal scope of 314 miles between refuelings.
They can be refueled in under 5 minutes. The U.S. Division of Energy's Fuel Cell Technology Program expresses that, starting in 2011, fuel cells accomplished 53–59% proficiency at one-quarter power and 42–53% vehicle productivity at full power and sturdiness of more than 120,000 km (75,000 mi) with under 10% degradation. In a 2017 Well-to-Wheels recreation examination that "didn't address the financial aspects and market imperatives", General Motors and its accomplices assessed that per mile voyaged, a fuel cell electric vehicle running on packed vaporous hydrogen delivered from petroleum gas could use about 40% less energy and radiate 45% fewer nursery gasses than an interior ignition vehicle.
In 2015, Toyota presented its first fuel cell vehicle, the Mirai, for $57,000. Hyundai presented the restricted creation Hyundai ix35 FCEV under a rent agreement. In 2016, Honda began renting the Honda Clarity Fuel Cell. In 2020, Toyota presented the second era of its Mirai image, improving fuel effectiveness and growing reach contrasted with the first Sedan 2014 model.
Criticism
A few reporters accept that hydrogen fuel cell vehicles won't ever turn out to be financially aggressive with other technologies or that it will require a long time for them to become profitable. Elon Musk, CEO of battery-electric vehicle creator Tesla Motors, expressed in 2015 that fuel cells for use in vehicles won't ever be industrially suitable in light of the shortcoming of delivering, moving, and putting away hydrogen and the combustibility of the gas, among other reasons.
In 2012, Lux Research, Inc. given a report that expressed: "The fantasy of a hydrogen economy ... is no closer". It presumed that "Capital expense ... will restrict appropriation to a simple 5.9 GW" by 2030, giving "an almost inconceivable hindrance to reception, besides in specialty applications". The investigation inferred that, by 2030, PEM fixed market will reach $1 billion, while the vehicle market, including forklifts, will arrive at an aggregate of $2 billion. Other examinations refer to the absence of a broad hydrogen foundation in the U.S. as a continuous test to Fuel Cell Electric Vehicle commercialization.
In 2014, Joseph Romm, the creator of The Hype About Hydrogen (2005), said that FCVs actually had not defeated the high fueling cost, absence of fuel-conveyance foundation, and contamination brought about by delivering hydrogen. "It would take a few marvels to conquer those issues at the same time in the coming decades." He reasoned that environmentally friendly power can't financially be utilized to make hydrogen for an FCV armada "either now or in the future." Greentech Media's investigator arrived at comparative resolutions in 2014. In 2015, Clean Technica recorded a portion of the hindrances of hydrogen fuel cell vehicles. So did Car Throttle.
A 2019 video by Real Engineering noticed that despite the presentation of vehicles that sudden spike in demand for hydrogen, utilizing hydrogen as a fuel for vehicles doesn't assist with diminishing fossil fuel byproducts from transportation. The 95% of hydrogen actually created from petroleum derivatives discharges carbon dioxide, and delivering hydrogen from water is an energy-devouring cycle.
Putting away hydrogen requires more energy either to chill it off to the fluid state or to place it into tanks under high tension and conveying the hydrogen to fueling stations requires more energy and may deliver more carbon. The hydrogen expected to move an FCV a kilometer costs roughly 8 fold the amount of the power expected to move a BEV the equivalent distance. A 2020 appraisal presumed that hydrogen vehicles are still just 38% proficient, while battery EVs are 80% efficient.
Markets and financial matters
In 2012, fuel cell industry incomes surpassed $1 billion market esteem around the world, with Asian pacific nations dispatching more than 3/4 of the fuel cell frameworks worldwide. However, as of January 2014, no open organization in the business had at this point become profitable. There were 140,000 fuel cell stacks delivered universally in 2010, up from 11,000 shipments in 2007, and from 2011 to 2012 overall fuel cell shipments had a yearly development pace of 85%. Tanaka Kikinzoku extended its assembling offices in 2011.
Approximately half of fuel cell shipments in 2010 were fixed fuel cells, up from about a third in 2009, and the four predominant makers in the Fuel Cell Industry were the United States, Germany, Japan, and South Korea. The Department of Energy Solid State Energy Conversion Alliance found that, as of January 2011, fixed fuel cells produced power at roughly $724 to $775 per kilowatt installed. In 2011, Bloom Energy, a significant fuel cell provider, said that its fuel cells created power at 9–11 pennies each kilowatt-hour, including the cost of fuel, upkeep, and hardware.
Industry bunches anticipate that there are adequate platinum assets for future demand, and in 2007, research at Brookhaven National Laboratory recommended that platinum could be supplanted by a gold-palladium covering, which might be less vulnerable to harming and consequently improve fuel cell lifetime. Another technique would utilize iron and sulfur rather than platinum.
This would bring down the expense of a fuel cell (as the platinum in a customary fuel cell costs around US$1,500, and a similar measure of iron expenses just around US$1.50). The idea was being created by an alliance of the John Innes Center and the University of Milan-Bicocca. PEDOT cathodes are invulnerable to monoxide poisoning.







0 Comments
Thanks for your feedback.